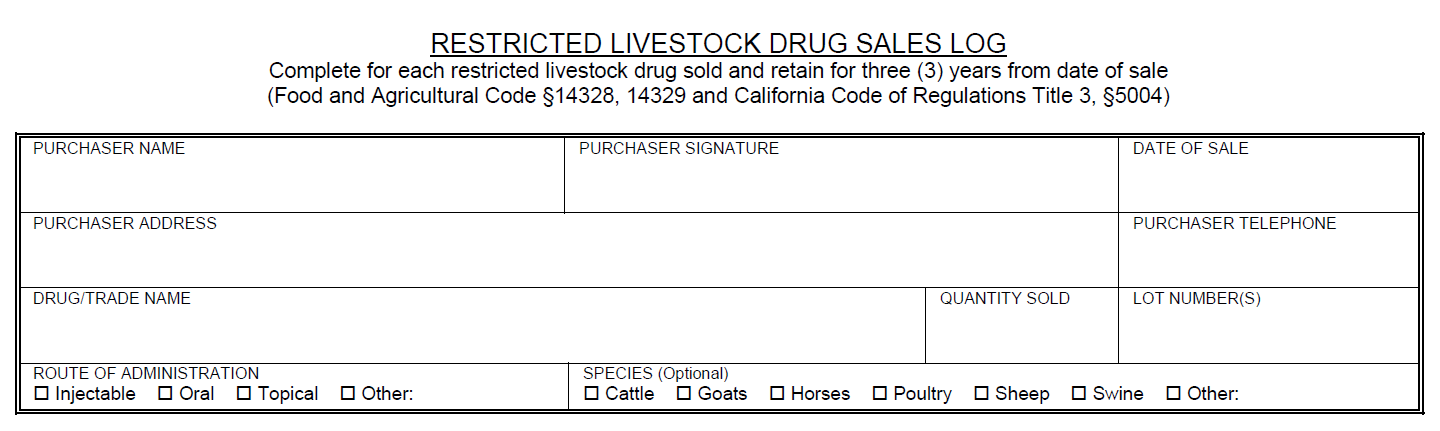
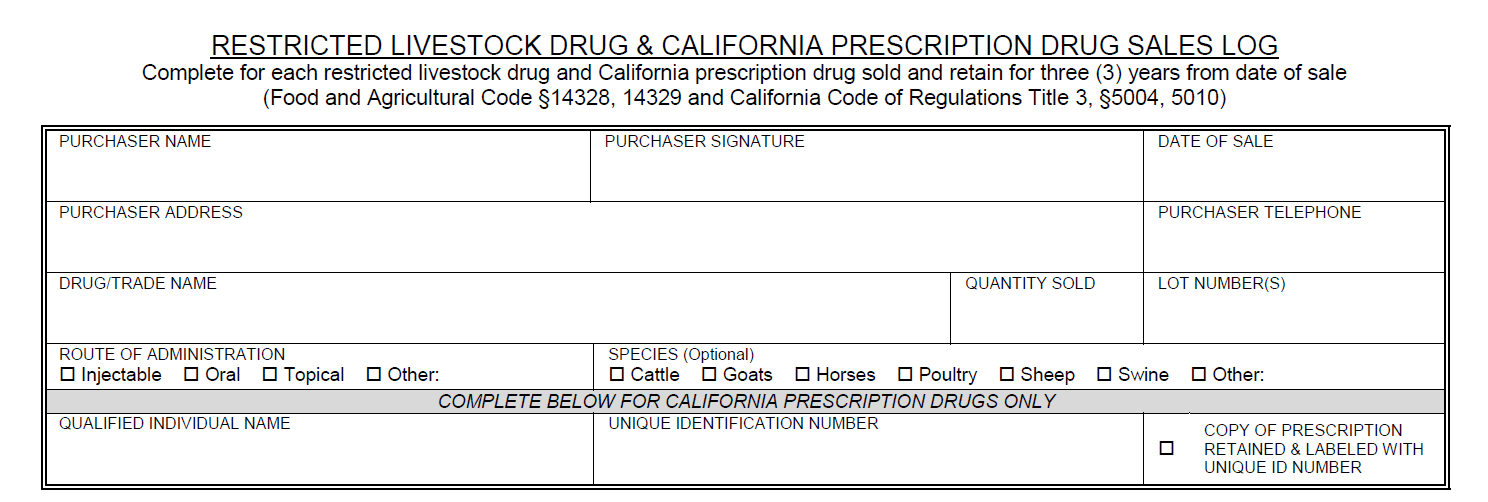
California Livestock Drug Qualified Individual and Licensed Retailer ResourcesCertain antimicrobial restricted livestock drugs changed status on January 1, 2018 to require a prescription for purchase and use in California. California prescription drugs (CA Rx drug) may only be dispensed by a Qualified Individual (QI) or pharmacist if a licensed retailer wants to sell CA Rx drugs.
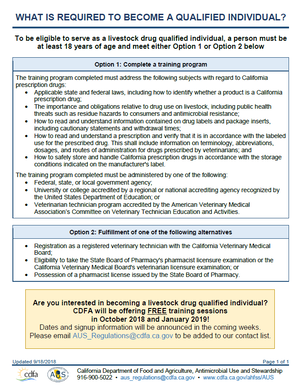
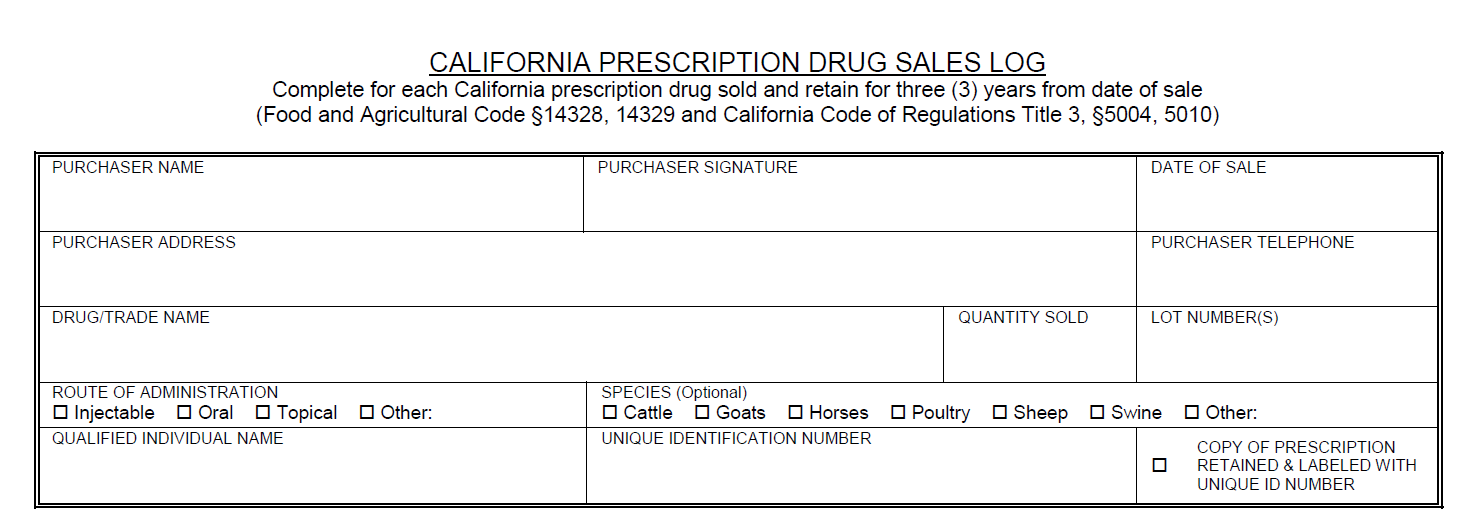
A QI can only dispense a drug if the prescription matches the FDA approved label exactly. The QI cannot break a drug down into individual units or dispense individual volumes/quantity of drug. A QI can only dispense prescription products in their original packaging. The quantity or volume to be dispensed does not necessarily have to be the exact volume that is required for the animal(s) treatment. Often times, the quantity of volume that will be written for and dispensed will be more than is required for the treatment plan. Additionally, the current treatment plan does not necessarily have to dictate the amount of drug dispensed. If the veterinarian anticipates the course will need to be repeated, etc., a larger volume/quantity can be dispensed as long as the quantity indicated corresponds to the quantity of product available on the market to be dispensed. The QI can dispense the amount indicated on the prescription as long as it corresponds to a marketed product. Additionally, the drug can be dispensed in multiple packages if the total quantity requested is larger then the volume of individual packages. For further explanation and examples click here. In order for a licensed retailer to sell CA Rx drugs, a consulting pharmacist or staff pharmacist will need to be employed. A pharmacist is needed in order to create store-specific written operating procedures as defined in Chapter 4, Section 5012 of the California Food and Agriculture code. Consulting and staff pharmacists can also aid in answering prescription questions and filling prescriptions that a QI is legally unable to fill. Please email AUS_Regulations@cdfa.ca.gov for additional information and to register for a QI training session.
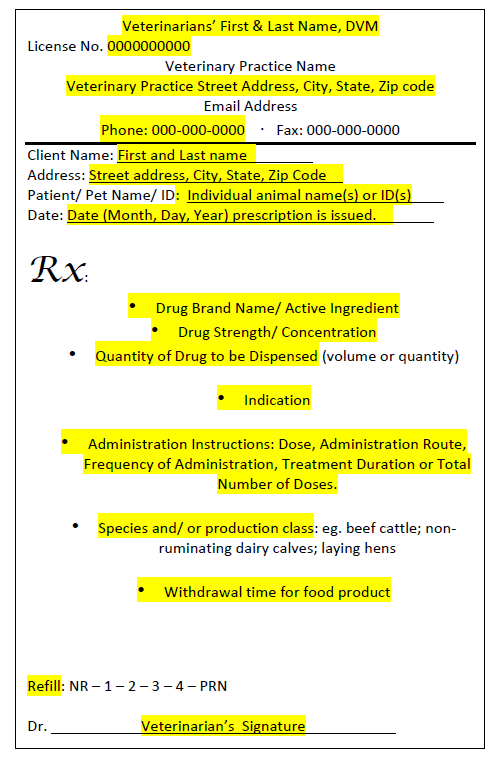
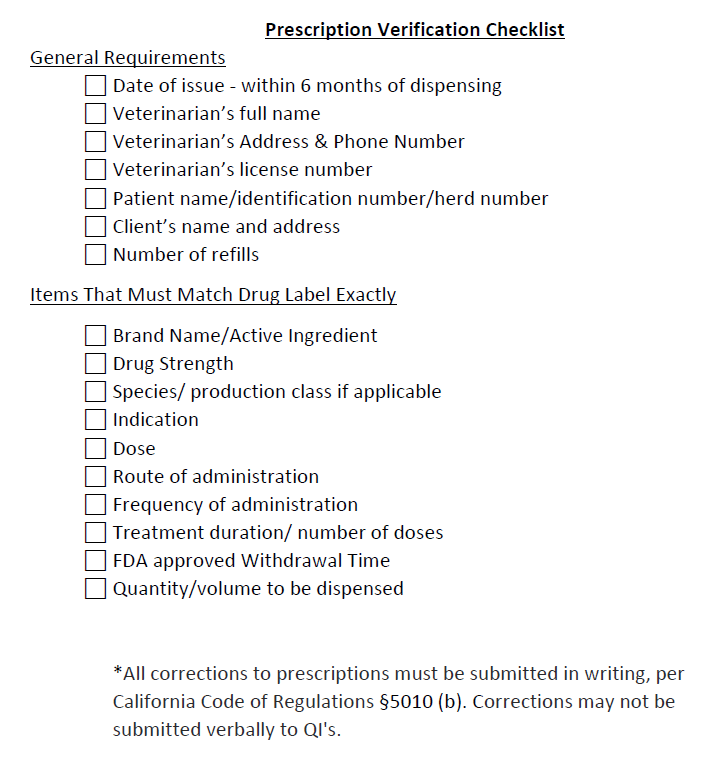
Prescription requirementsThe following highlighted portions need to be specified on a prescription if a QI is to dispense the CA Rx Drug:
|
Other abbreviationsq = every h or hr = hours NR = No additional refills of prescription allowed PRN= refill prescription as needed. |
Resources
|
| ||||||||||||
|
| ||||||||||||
|
| ||||||||||||||||||
| ca_rx.pdf | |
| File Size: | 36 kb |
| File Type: | |
| rld.pdf | |
| File Size: | 113 kb |
| File Type: | |
| rld___ca_rx.pdf | |
| File Size: | 37 kb |
| File Type: | |