VFDs for Vets: What these changes mean to veterinarians
Responsibilities
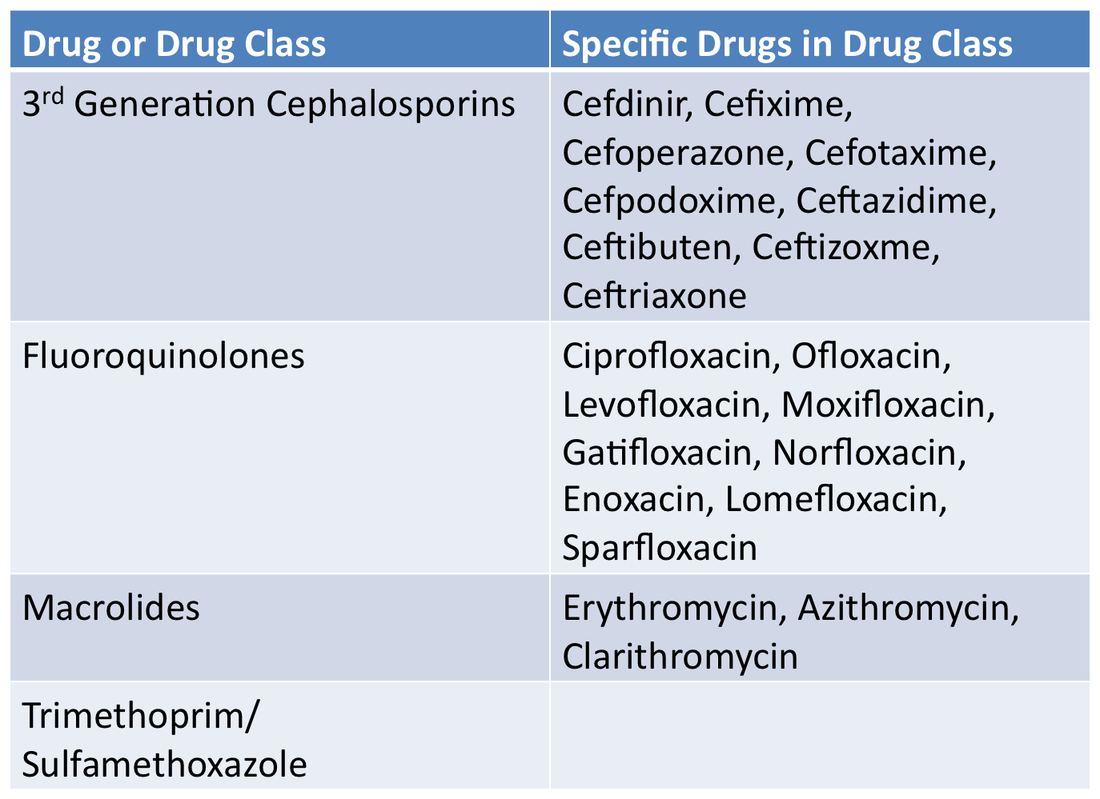
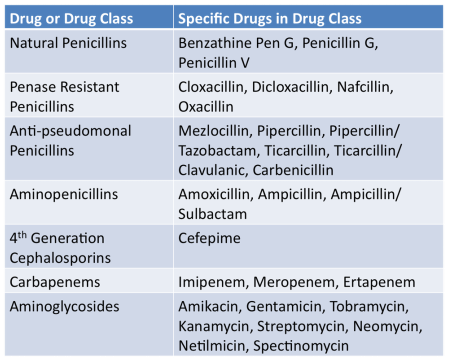
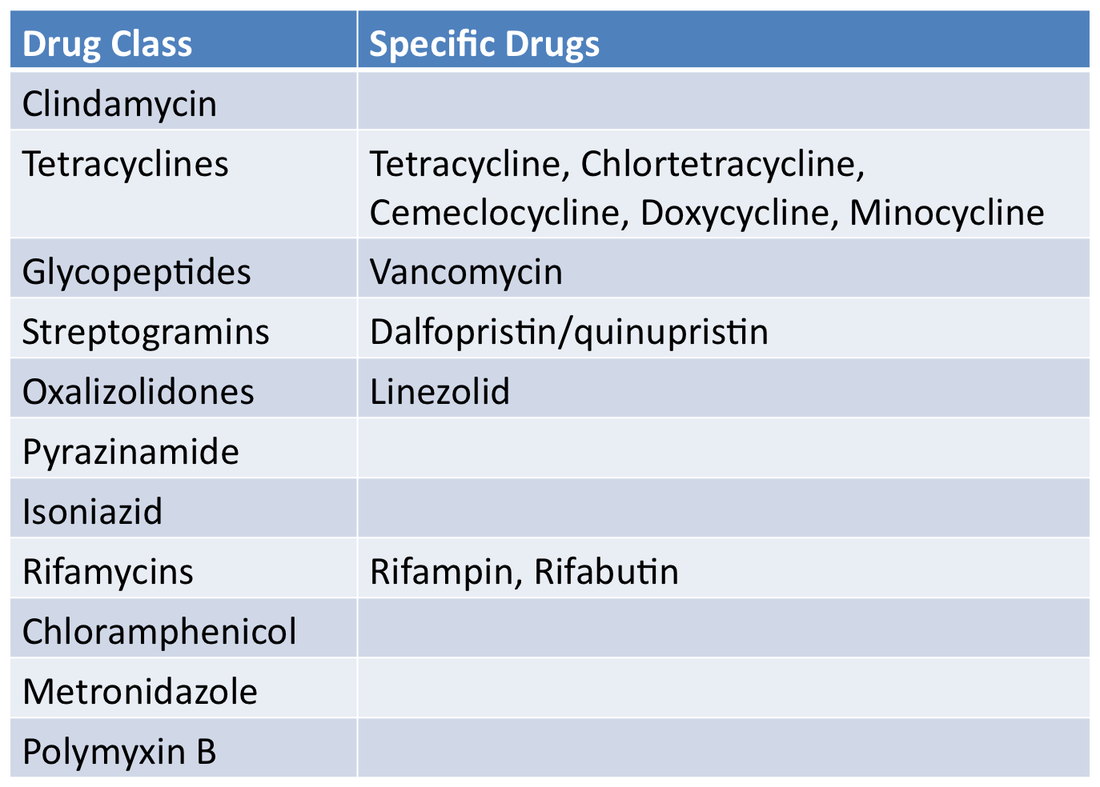
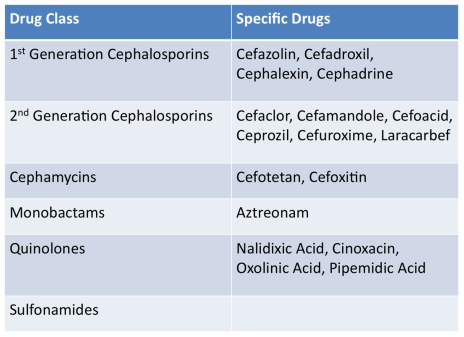
Medically Important AntimicrobialsBelow are FDA's ranking of Antimicrobial drugs important for use in human medicine as listed in the appendix of GFI #152. They are ranked as Critically Important, Highly Important and Important. Any drug included in any of these categories will be affected by these new regulations and will require veterinarian oversight for their use in the feed or water of livestock.
|
Required Information on VFD order
|
VCPR: Federal vs State DefinitionSome states have their own definition of a VCPR. Veterinarians in those states will abide by the State definition. If the state does not have a defined VCPR, the federal definition applies (21 CFR 530.3(i). States following the federal definition: Alabama, Alaska, Arkansas, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Kansas, Maryland, Massachusetts, Michigan, Montana, New Jersey, New York, Pennsylvania, Rhode Island, South Dakota, Vermont, Washington, West Virginia, Wisconsin. States following their state definition: Arizona, California, Colorado, Idaho, Illinois, Iowa, Kentucky, Louisiana, Maine, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Hampshire, New Mexico, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, South Carolina, Tennessee, Texas, Utah, Virginia, For more details click here. |
Critically ImportantHighly ImportantImportantNot Important for Human MedicineDrugs in these classes will not be affected by the new regulations.
|
|
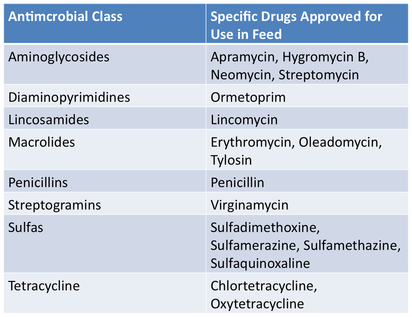
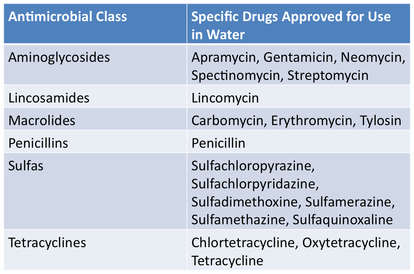
Drugs Affected by this LegislationA number of feed-use medications and water-use medications were affected by these guidelines December 2016. A general list of affected antimicrobials is below. A product specific list may be found on the FDA site.
Affected Feed-Use AntimicrobialsAffected Water-Use Antimicrobials |