What does California SB 27 mean to veterinarians?
All antimicrobials deemed important for use in human medicine (MIA) will require a prescription from a veterinarian with a valid veterinary-client-patient relationship. California defines a valid VCPR in Section 2032.1 of Title 16 of the California Code of Regulations.
The main difference with this bill and the federal regulation is that it applies to all routes of administration, including injectable, intramammary, and topical antibiotics, as well as antibiotics meant for water or feed. This has resulted in those products that are currently available in feed stores as an over-the-counter (OTC) product becoming prescription only in the state of California (CA RX Drug).
This bill also specifies that prophylaxis use of MIA may only be used if an elevated risk for disease/infection exists as determined by a veterinarian.
The main difference with this bill and the federal regulation is that it applies to all routes of administration, including injectable, intramammary, and topical antibiotics, as well as antibiotics meant for water or feed. This has resulted in those products that are currently available in feed stores as an over-the-counter (OTC) product becoming prescription only in the state of California (CA RX Drug).
This bill also specifies that prophylaxis use of MIA may only be used if an elevated risk for disease/infection exists as determined by a veterinarian.
Veterinary Written Order- 16 CCR 2032.2
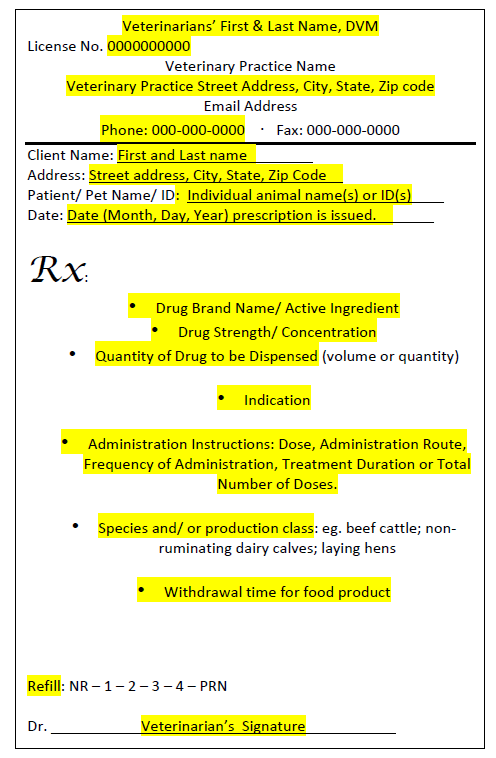
In order for a Qualified Individual (QI) to dispense a California prescription drug (CA Rx drug) through a licensed retailer, the prescription issued must match the drug label exactly and the prescription must be filled out correctly with all necessary component highlighted below. Additionally the prescription is only valid within 6 months of issue.
|
The following information must be written on the prescription by the veterinarian in order for a QI to dispense CA Rx drugs.
|
Corrections to a prescription must be submitting in writing to a QI and may not be submitted verbally. Per California Code of Regulations Title 3 §5010 (b), only a licensed pharmacist may dispense prescriptions via a verbal order correction.
The quantity or volume to be dispense must be indicated on the prescription in order for the CA Rx drug to be dispensed. The QI may not break down or measure out specific amounts of drug (repackage), they may only dispense drugs as they are originally packaged. Therefore, the quantity/volume to be dispensed must be the same as what the product is packaged as (or multiples of).
The quantity or volume to be dispensed does not necessarily have to be the exact volume that is required for the animal(s) treatment. Often times the quantity of volume that will be written for and dispensed will be more than is required for the treatment plan.
Example:
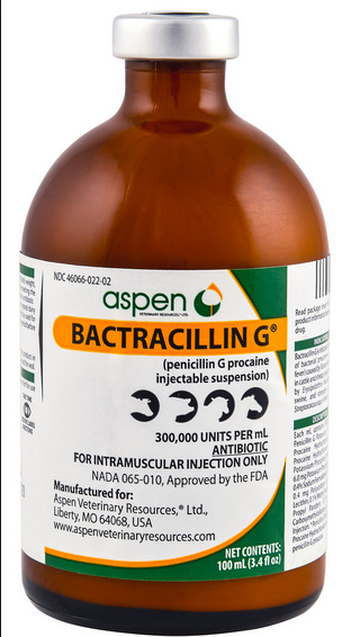
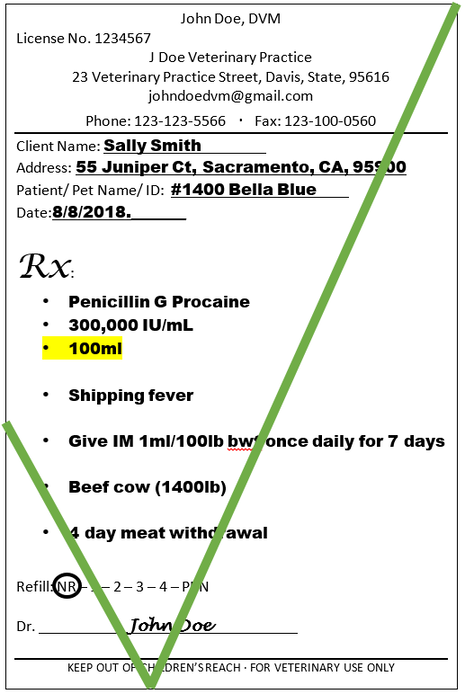
If the prescription is written for a volume of 98mL but the product only comes as 100mL, the QI cannot withdrawal 98mL from the original packaging and dispense 98mL.
The quantity or volume to be dispensed does not necessarily have to be the exact volume that is required for the animal(s) treatment. Often times the quantity of volume that will be written for and dispensed will be more than is required for the treatment plan.
Example:
If the prescription is written for a volume of 98mL but the product only comes as 100mL, the QI cannot withdrawal 98mL from the original packaging and dispense 98mL.
The prescription would need to be corrected by the veterinarian to reflect a volume that the products is commercially supplied in.
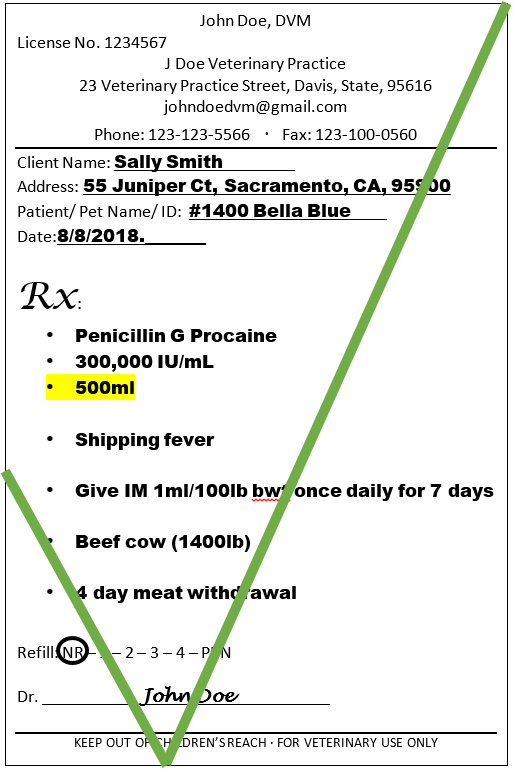
Additionally, the current treatment plan does not necessarily have to dictate the amount of drug dispensed. If the veterinarian anticipates the course will need to be repeated, etc., a larger volume/quantity can be dispensed as long as the quantity indicated corresponds to the quantity of product available on the market to be dispensed.
Example:
Example:
The Qualified individual can dispense the amount indicated on the prescription as long as it corresponds to a marketed product. Additionally, the drug can be dispensed in multiple packages if the total quantity requested is larger then the volume of individual packages.
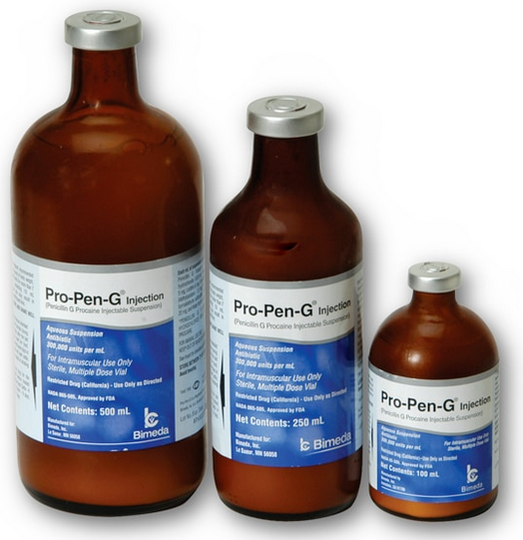
Example: If a prescription is indicating 500ml penicillin to be dispensed. That drug can be dispensed in 3 different ways:
a.) 1 x 500ml bottle
b.) 2 x 250ml bottles
c.) 5 x 100ml bottles
Example: If a prescription is indicating 500ml penicillin to be dispensed. That drug can be dispensed in 3 different ways:
a.) 1 x 500ml bottle
b.) 2 x 250ml bottles
c.) 5 x 100ml bottles